Ultra-diluted Toxicodendron pubescens attenuates proinfammatory cytokines and ROSmediated neuropathic pain in rats
- News
-
Nov 06
- Share post
Despite the availability of multiple therapeutic agents, the search for novel pain management of neuropathic pain is still a challenge.Oxidative stress and inflammatory signaling are prominently involved in clinical manifestation of neuropathic pain. Toxicodendron pubescens, popularly known as Rhus Tox (RT) is recommended in alternative medicines as an anti-inflammatory and analgesic remedy. Earlier, we reported anti-inflammatory, anti-arthritic and immunomodulatory activities of Rhus Tox.
In continuation, we evaluated antinociceptive efficacy of Rhus Tox in the neuropathic pain and delineated its underlying mechanism. Initially, in-vitro assay using LPS-mediated ROS-induced U-87 glioblastoma cells was performed to study the effect of Rhus Tox on reactive oxygen species (ROS), anti-oxidant status and cytokine profile. Rhus Tox decreased oxidative stress and cytokine release with restoration of anti-oxidant systems.
Chronic treatment with Rhus Tox ultra dilutions for 14 days ameliorated neuropathic pain revealed as inhibition of cold, warm and mechanical allodynia along with improved motor nerve conduction velocity (MNCV) in constricted nerve. Rhus Tox decreased the oxidative and nitrosative stress by reducing malondialdehyde (MDA) and nitric oxide (NO) content, respectively along with up regulated glutathione (GSH), superoxide dismutase (SOD) and catalase activity in sciatic nerve of rats. Notably, Rhus Tox treatment caused significant reductions in the levels of tumor necrosis factor (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) as compared with CCI-control group.
Protective effect of Rhus Tox against CCI-induced sciatic nerve injury in histopathology study was exhibited through maintenance of normal nerve architecture and inhibition of inflammatory changes. Overall, neuroprotective effect of Rhus Tox in CCI-induced neuropathic pain suggests the involvement of anti-oxidative and anti-inflammatory mechanisms.
Department of Pharmacology, R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur- 425405, Dist. Dhule, Maharashtra, India. 2School of Biotechnology, Kalinga Institute of Industrial technology (a deemed to be University), Campus-11, Patia, Bhubaneswar, Odisha, Pin-751024, India. 3SVKM’s Institute of Pharmacy, Dhule-424001, Dist-Dhule, Maharashtra, India. 4 Janmangal Homeopathy and Wellness Centre, Bopal, Ahmedabad, Gujarat, 380058, India. 5 Department of Pharmacology & Therapeutics, College of Medicine & Health Sciences, UAE University, Al Ain, UAE. Correspondence and requests for materials should be addressed to C.R.P. (email: xplore.remedies@gmail.com) or S.O. (email: shreeshojha@uaeu.ac.ae) or C.N.K. (email: cnkundu@kiitbiotech.ac.in) inhibitors and calcium channel ligands in the treatment of neurogenic pain. However, these agents do not sufce the targets of managing of neuropathic pain. Te efcacy of available anti-neuropathic drugs is limited due to occurrence of several side efects and inadequate or delayed pain relief Te need to discover novel treatment modalities for neuropathic conditions still prevails.
Various factors contribute to the peripheral sensitization and initiation of neuropathic pain. Raised oxidative stress, increased vascular permeability and release of diferent infammatory mediators including substance P and calcitonin gene related peptide produced by nociceptive terminals, formation and/or release of bradykinin, prostaglandins, growth factors and cytokines leads to the occurrence of neuropathic pain7
. Recently, the anti-infammatory and antioxidant agents like N-acetyl carnitine and alpha lipoic acid are being investigated as add-on medications for the management of neuropathic pain.
Natural products or complementary and alternative medicines are frequently used to manage the chronic neurological disorders like neuropathic pain . Few preclinical studies on homeopathic remedies have also demonstrated the efcacy of homeopathic remedies in the management of neuropathic pain9,10. Evidence based surveys have revealed that patients with chronic pain prefer to use herbal treatments for these painful condition. Several pre-clinical and clinical studies have reported the benefcial efect of medicinal plants in the therapy of painful neuropathy. Toxicodendron pubescens P. Mill belonging to family anacardiaceae is known as Rhus toxicodendron or Rhus tox (RT) in alternative medicines. RT is commonly used homeopathic remedy for the management of infammatory conditions, rheumatic pain, typhoid type fever and mucous membrane afections. Experimental studies have shown that RT possesses immunomodulatory, anti-infammatory anti-arthritic and anti-melanoma. Recently, a prospective observational study in breast cancer patients has shown that RT may decrease joint pain and stifness in women with early breast cancer26.
Surgical lesion of peripheral nerves in experimental animals are usually performed to produce the animal models resembling human neuropathic pain27. In this regard, chronic constriction injury (CCI) model appears to be well established and frequently used animal model for the study of neuropathic pain27,28. In this model, unilateral loose ligation of sciatic nerve mimics the pathological neuropathic pain state in humans29. In this report, wedemonstrated antinociceptive efects of RT in neuropathic pain using in-vitro and in-vivo assays. Initially, in-vitro study using U-87 primary glioblastoma cell line was executed to delineate the efect of RT on LPS-induced ROS production, anti-oxidant status and cytokine levels. Furthermore, in-vivo efcacy of RT was assessed in neuropathic pain using well characterized animal model of sciatic nerve constriction injury-induced neuropathic pain in rats. Various behavioral testing paradigms (cold, warm and mechanical allodynia), physiological parameter(MNCV), biochemical estimations (MDA, NO, GSH, SOD, catalase, TNF-α, IL-1β, IL-6) and histopathology was executed to explore the underlying analgesic mechanisms of RT in the experimental neuropathic pain. Gabapentin served as a positive control in this study.
Results
Efect of RT on LPS-induced oxidative stress in U-87 glioblastoma cells. The treatment of U-87 cells with LPS resulted into a marked increase in the cell viability. Whereas, treatment of cell with RT at tested concentrations decreased LPS-mediated ROS induced cell viability in a dose dependent manner (Fig. 1A). There was no considerable cytotoxicity due to RT treatment in absence of LPS stimulation (data not shown). To overrule the interference of cytotoxicity of RT, we tested the concentrations of RT which were devoid of any prominent effect on the cell viability. Stimulation of cells with LPS has resulted into decreased levels of SOD and catalase activity in primary glioblastoma U-87 cells. But afer addition of RT (1×10−8; 1×10−12; 1×10−24 and 1×10−30) in LPS pre-treated cells ofered a signifcant increase in SOD (***P<0.001) and catalase activity (**P<0.01) as compared to the LPS-treated control cells. RT exhibited dose dependent efect on these anti-oxidant systems (Fig. 1B,C).
The level of ROS production in U-87 cells afer LPS-stimulation was evaluated through fow cytometric analysis by DCFH-DA staining. Following LPS stimulation, approximately 70% of U-87 cells were = revealed as ROS positive. The significant (***p<0.001) and concentration dependent = decrease in ROS positive cells were observed afer treatment of LPS-pre-treated cells with RT (1×10−8; 1×10−12; 1×10−24 and 1×10−30). The percentage of ROS positive cells was found to be reduced in RT (1×10−8; 1×10−12; 1×10−24 and 1×10−30) treated cells, respectively. Te H2O2 was used as a positive control for ROS generation (Fig. 1D,K).
Effect of RT on LPS-induced pro-inflammatory cytokine expression in U-87 glioblastoma cells. To check the efect of RT on pro-infammatory cytokines, the level of TNF-α, IL-1β, IL-6 and IL-10 were measured in LPS pre-treated U-87 cells. Treatment of cells with LPS (500ng/ml for 20 min) resulted into signifcant increase in the level of various cytokines as compared to untreated cells. As compared to LPS-control, the treatment of LPS-pre-treated cells with RT (1×10−8; 1×10−12; 1×10−24 and 1×10−30) resulted into the dose dependent and signifcant decrease in the levels of TNF-α (*P<0.05), IL-1β (*P<0.05), IL-6 (**P<0.01) and IL-1β (**P<0.01). Interestingly, the RT treatment almost brought down the cytokines level to basal level.
Efect of RT on cold, warm and mechanical allodynia in CCI-induced neuropathic pain. Effect of RT on cold, warm and mechanical allodynia in rats during CCI-induced neuropathic pain is depicted as Fig. 3A–C. Constriction of sciatic nerve in the animals caused a signifcant development of cold allodynia (Fig. 3A), warm allodynia (Fig. 3B) and mechanical allodynia (Fig. 3C) as compared with the sham operated group, evaluated through cold water test, warm water test and Von Frey hair test, respectively.
The paw withdrawal latency (PWL) with respect to cold allodynia was signifcantly decreased in CCI-control rats as compared with the sham operated rats (8.7 ± 1.1 sec Vs. 16 ± 0.4 sec, P < 0.001). RT (1 × 10−12 dilution) signifcantly elevated the PWL as compared with the CCI-control animals (13±0.3 sec Vs. 8.7±1.1 sec; F (5, 20)=5.641, P<0.001). Tese efects were similar and less potent than gabapentin (60 mg/kg/day, p.o.).
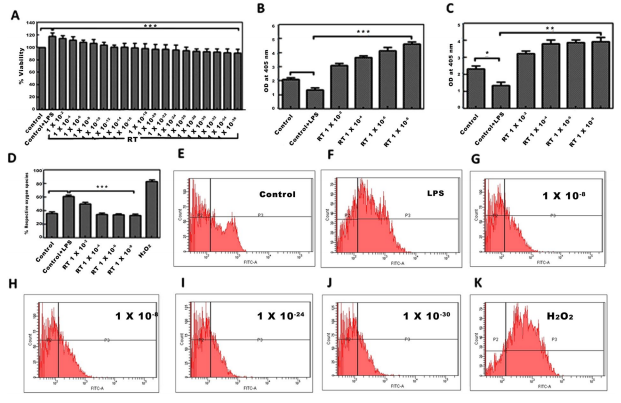
Figure 1. Efect of RT on LPS mediated ROS, SOD and catalase activity in U-87 cells. (A) MTT cell viability assay afer RT exposure in LPS-pre-treated U-87 cells. ***p<0.001. (B) Biochemical detection of SOD, and (C) catalase following treatment with RT in LPS pre-treated U-87 cells. (D) LPS pre-treated (500ng/ml for 20min) cells were treated with various concentrations of RT prior to estimation of ROS. Graphical representation of ROS positive population analyzed by DCFH-DA staining using fow cytometer (E–K) were the graphical representation of cell count in Control, Control+LPS, RT (1×10−8, 1×10−12, 1×10−24 and 1×10−30) and H2O2 (positive control) treated U-87 cells, respectively. Data represents mean±S.E.M. of 3 independent experiments. **p<0.01 and ***p<0.001 as compared to LPS treated cells.
Gabapentin increased the PWL in response to cold stimuli up to 19±0.4 sec as compared with CCI control group 16±0.5 (P<0.001) Fig. 3A. Efect of RT on warm allodynia following CCI surgery is represented as Fig. 3B. CCI induced a signifcant decrease in PWL (11±0.3 sec) as compared with the sham operated group and normal group. RT treatment resulted in a signifcant elevation of PWL up to 14±0.5 sec as compared to the CCI control. group (P<0.001) as shown in Fig. 3B. We determined the paw withdrawal threshold (PWT) using Von Frey hairs to assess the mechanical allodynia in rats. Te PWT in CCI control rats (6.9±0.7 g) was signifcantly lower as compared to the rats of sham group. RT and gabapentin treatments signifcantly increased the PWT as compared to the CCI-control rats as shown in Fig. 3C.
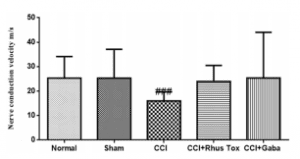
Effect of RT on MNCV in CCI-induced neuropathic pain in rats. The MNCV was estimated on the 14th day. CCI induced a signifcant reduction in the MNCV. As compared to the sham operated group having MNCV equal to 23.2±3.8mm/sec the CCI induced rats had MNCV of 15.9±2.1mm/sec (P<0.001). RT and gabapentin treatments increased the MNCV up to 23.9±2.07 and 25.3±5.92mm/sec respectively. However, these efects of RT and gabapentin were statistically non-signifcant (Fig. 4)
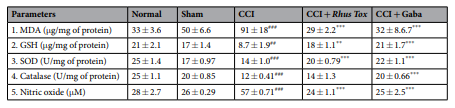
Effect of RT on oxidative stress in CCI-induced neuropathic pain. The extent of lipid peroxidation in the nerve homogenate measured as MDA was signifcantly higher in ligated sciatic nerve as compared with sham operated group (91±18 Vs. 50±6.6μg/mg of protein; P<0.001). Similarly, the nitric oxide level was significantly elevated in CCI-control group as compared to the sham group (P<0.001). CCI induced oxidative stress caused significantly reduced levels of GSH and inhibited the SOD and catalase activities in the nerve homogenates as compared with the sham operated rats. Oral administration of RT for 14 days signifcantly attenuated the CCI-induced increase in MDA and NO levels. RT treatment also restored the levels of GSH and SOD activity. The restorative efect of RT on the catalase activity was not statistically signifcant. Gabapentin notably ameliorated the oxidative stress in CCI-induced rats. Moreover, the effects of RT in reducing the MDA and NO levels were comparable with the effects of the standard drug gabapentin (Table 1).
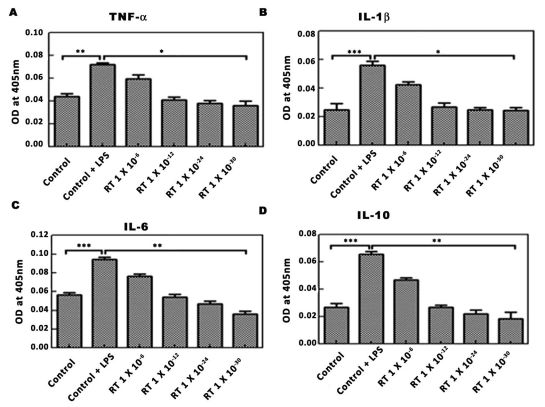
Figure 2. Effect of RT on LPS stimulated pro-infammatory cytokines in U-87 cells. LPS pre-treated (500ng/ml for 20min) cells were treated with various concentrations of RT before the estimation of cytokines. (A–D) Are the graphical representation of optical density of cell supernatants at 405nm for TNF-α, IL-1β, IL-6 and IL-10. Data represents mean±S.E.M. of 3 independent experiments. **p<0.01 and ***p<0.001 as compared to LPS treated cells.
Effect of RT on pro-infammatory cytokines in CCI-induced neuropathic pain. Te CCI induced a notable rise in the levels of pro-infammatory cytokines. Te tissue levels of TNF-α, IL-6 and IL-1β in the CCI-induced group were 56 ± 2.6, 44 ± 4.8 and 64 ± 6.8 pg/mg respectively. Whereas, in the sham operated group the levels of TNF-α, IL-6 and IL-1β were 26±0.4, 22±0.3 and 22±0.3 pg/mg. Te CCI-induced group had signifcantly higher levels of proinfammatory cytokines in the nerve tissue (P<0.001). RT treatment for 14 days remarkably decreased the levels of TNF-α to 25±1.1pg/mg, IL-6 to 20±1.4pg/mg and for IL-1β up to 20±1.4pg/mg of protein (p<0.001). Tese efects of RT on the pro-infammatory cytokines were similar to the effects exerted by gabapentin (Fig. 5). RT exerted more prominent inhibitory efect on IL-1β levels as compared to the efect of gabapentin.
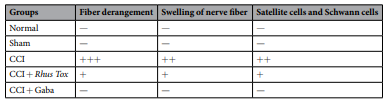
Effect of RT on CCI-induced histological alterations in the sciatic nerve. The CCI induced characteristic histological changes in sciatic nerve. Alterations like nerve fbre swelling, infammatory cell inflteration, fbre derangement and activation of neuroglial cell like satellite cells and Schwann cells suggest the damage of the sciatic nerve. Treatment of animals with RT or gabapentin protected the nerve from CCI-induced changes. The nerve samples from rats receiving these treatments revealed that both RT and gabapentin had reduced structural derangements, along with inhibition nerve fber and neuroglial cell swelling (Fig. 6 and Table 2). The longitudional sections of sciatic nerve of normal, sham, CCI-control, CCI+RT (1×1012; 0.1ml/day, p.o.) treated and CCI+gabapentin (60 mg/kg, p.o) treated groups are shown as Fig. 6, respectively.
Histopathology study of sections from normal rats demonstrated the normal architecture of sciatic nerve with no infammatory perturbations. Sections obtained from the sham operated rats also revealed normal structure with no marked changes. However, the CCI-control rats showed nerve fbre swelling, derangement of fbre architecture, infammatory cell infltration along with alterations in satellite cells and schwann cells (Table 2). Treatment with RT (1 × 10−12; 0.1 ml/day, p.o.) or gabapentin (60 mg/kg/day, p.o.) exibited ameliorative effect against the CCI-induced changes in the sciatic nerve of rats as evident by reduction in infammatory alterations and structural derangement (Magnifcation 100×, scale bar, 1000 µm).
Discussion
CCI-induced neuropathic pain in rats represents characteristic painful behaviors like hyperalgesia and allodynia, thus validating its suitability for the evaluation of anti-neuropathic drugs29–31. In this model, the neuropathic pain is caused by a primary lesion and dysfunction of the somatosensory nervous system3,14. RT ultra-dilutions have been consistently proved to possess analgesic, anti-infammatory22 and immunomodulatory21 activities.
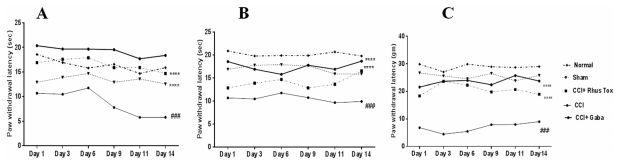
Figure 3. RT improved the cold, warm and mechanical stimuli induced allodynia in CCI induced neuropathic pain in rats. Data were expressed as the mean±SEM (n=8). Statistical signifcance was determined by repeated measures ANOVA (analysis of variance) followed by Bonferroni’s post hoc test, ###p<0.001 as compared to sham operated group. While, the statistically signifcant diference in RT and gabapentin administered groups as compared with CCI-control group was represented as ***p<0.001. Te p<0.05 was considered statistically signifcant.

Experimental models. We investigated the efects of RT using in vitro model of LPS-induced oxidative stress and cytokine release from the U-87 glioblastoma cell culture to determine the exact ultra-dilution which inhibits the induced oxidative stress. Te ultra-dilution that was found to be the most efective in reducing oxidative stress in nerve cells was then tested for the anti-neuropathic efcacy against CCI-induced pain. The efcacy of RT to inhibit LPS-induced oxidative stress in the U-87 cells was evident in our study. RT 1×10−12 and 1×10−15 dilutions reduced the percentage of ROS positive cells and also decreased the expression of pro-infammatory cytokines (TNF-α, IL-1β, IL-6 and IL-10) along with decrease in the levels of SOD and catalase. Tese fnding signify the in-vitro potential of RT ultra-dilutions to protect the neuronal cells from induced oxidative stress. Dos Santos et al. 19 has reported that the anti-infammatory activity of RT is maximum when its homeopathic dilution corresponding to 1×10−12 dilution was tested against the carrageenan-induced pawedema in rats. Similar pattern of maximal efcacy was found in our earlier studies on RT where we evaluated its anti-infammatory, immunomodulatory and anti-arthritic efect21–24. Te present in vitro fndings indicating the effectiveness of RT in reducing the oxidative stress and pro-infammatory cytokine release are in congruence with these reports.
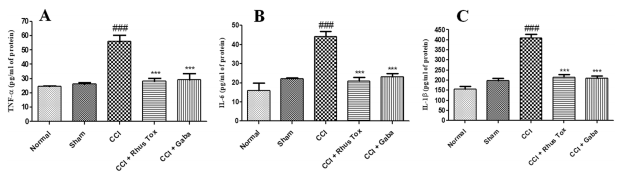
Figure 5. RT reduced the release of pro-infammatory cytokines in CCI-induced neuropathic pain. Data are represented as mean±SEM (n=8). Statistical signifcance was analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. p<0.05 was considered statistically signifcant. Statistical diference of CCI-control group as compared with sham operated group was represented as ###p<0.001. Whereas, the statistical diference of RT or gabapentin treated group as compared to CCI-control group was represented as, ***p<0.001.
RT inhibitted the CCI-induced histological alterations in the longitudional sections of sciatic nerve in rats.
Across the 14 days post CCI surgery, the rats exhibited characteristic behavioural symptoms of neuropathic pain similar to the clinical symptoms. The paw withdrawal threshold (PWT) and paw withdrawal latency (PWL) against mechanical and thermal stimuli were used for determination of the neuropathic pain. RT treatment efectively elevated these pain thresholds indicating the reduced neuropathic pain sensations. In line with the published data indicating the anti-infammatory and analgesic activities, our study proves that RT also efectively reduces the neuropathic pain.
The CCI induces ischemic hypoxic imbalance in the secondary metabolites in affected nerve fbres and produces oxidative stress32,33. Degeneration of nerve fbre and decreased nerve energy is reported to reduce the MNCV. RT ameliorated the nerve damage mediated reduction in the MNCV following CCI in sciatic nerve indicating its neuroprotective efect. Oxidative stress and infammation are interconnected events leading to nerve injury and persistent pain34,35. Various plant extracts, their isolated fractions or phytoconstituents have been reported to exert their anti-neuropathic activity through the anti-infammatory and anti-oxidant mechanisms35–40. The concentrations of MDA and NO are commonly measured to study the extent of reactive oxygen species34. ROS like superoxide, NO and peroxynitrite have an important role in the development of neuroinfammatory responses. Increase in lipid peroxidation following CCI of sciatic nerve has also been reported. Efcacy of free radical scavengers towards the reduction of lipid peroxidation suggests the involvement of ROS in the nerve sensitization and production of allodynia.
NO is the vital signaling molecule which has signifcant role in the peripheral and central pain. Involvement of endogenous nitric oxide in the development of CCI-induced neuropathic pain is well documented in earlier studies. Signifcant rise in the levels of NO in sciatic nerve suggested the up regulation of NO and its role in the production and maintenance of neuropathic pain. In the present study signifcant increase in the levels of MDA and NO were observed in the chronically constricted nerve. It denotes elevated damage to the macromolecules such as proteins and lipids which may result into the neuropathic pain. Chronic administration of RT resulted in the decreased levels of MDA and NO indicating the anti-lipid peroxidation activity along with inhibition of nitrosative tissue damage and successive neuropathic pain.
Our fndings are in line with previous studies reporting the decline in GSH content of sciatic nerve following CCI in rats46. Catalase and SOD are main anti-oxidant enzymes that help to scavenge the free radicals and ofer protection against oxidative stress. In our study, decrease in the level of catalase and SOD after CCI in the rats are indicative of increased oxidative stress or declined anti-oxidant defense mechanisms. These results are in tune with earlier reports and reinforce the contribution of oxidative stress in the development of neuropathic pain47–49. Oxidative stress mediated decline in the levels of GSH, SOD and catalase were restored in the CCI rats by chronic treatment with RT ultra-dilution (1×10−12). Tus, RT mediated decrease in the CCI-induced oxidative stress could be one of the mechanisms involved its antinociceptive activity in neuropathic pain.
Diferent cytokines including TNF-α IL-1β, and IL-6 are released following the nerve injury and contribute to the development of neuropathic pain. Peripheral nerve damage induces release of TNF-α rapidly from macrophages, Schwann cells and mast cells. TNF-α is associated with decline in pain threshold and anti-TNF-α treatments are reported to alleviate the CCI-induced pain in rats51. In this study, we observed a signifcant decrease in the TNF-α level in sciatic nerve in the group treated with RT as compared with CCI-control group.
This fnding revealed that RT modulated the neuro-infammation in peripheral nerve through its anti-TNF-α action and it could be one of its anti-nociceptive mechanisms. IL-1β is known to be expressed in nociceptive neurons and it has important role in several pain models52,53. Te IL-1β increases the neuropathic pain sensitization through its action on the adjacent neurons34. We observed a signifcant increase in the production of IL-1β in sciatic nerve afer CCI in rats. Treatment of animals with RT inhibited IL-1β production which may account for its antinociceptive efect. IL-6 is a pro-infammatory cytokine having important role in the development of infammatory and neuropathic pain following peripheral nerve injury. Results of present study revealed that RT has ability to decrease the IL-6 level which signifes the anti-IL-6 efect of RT in CCI-induced neuropathic pain model. Overall fndings through biochemical estimation denote that RT favorably altered the cytokine profle in the experimental model of neuropathic pain.
In summary, this study substantiated the antinociceptive potential of RT ultra-dilutions in the validated model of CCI-induced peripheral neuropathic pain. Interestingly, RT was found to be efcacious in reducing not only the thermal nociception but also the mechanical allodynia in rats. RT demonstrated notable anti-oxidant and anti-infammatory efects in the sciatic nerve and exhibited potential free radical scavenging activity that would reduce its anti-neuropathic activity. Attenuated oxidative stress and infammatory pathways may contribute to the therapeutic potential of RT in the treatment of neuropathic pain. Although, the results of present study suggested the anti-neuropathic efect of RT, further pre-clinical and clinical studies are warranted to confrm these effects. Several other biochemical mechanisms may be involved in RT mediated anti-neuropathic efect. Results present study are suggestive of the anti-nociceptive efect of RT against neuropathic pain and deserve further validation of its efectiveness in various painful conditions.
Methods
Chemicals. The authenticated dried powder of leaves of Rhus Tox (Family: Anacardiaceae) was obtained from Homeopathic Pharmacopoeia laboratory, Ghaziabad, Uttar Pradesh, India. Gabapentin was gifed by Mylan laboratories, India. Cytokine ELISA Ready SET-Go kits for TNF-α (Cat: 837324-22: Batch No. E09479-1645), IL-1β (Cat: 887013-22; Batch No. E09323-1645) and IL-6 (Cat: 837064-22: Batch No. E09358-1645) were purchased from e-Biosciences Incorporation, USA. Lipopolysaccharide (LPS) from Escherichia coli O55:B5 (Cat: L2880; Lot No. 025M4040V) was purchased from Sigma-Aldrich, USA.
Preparation of RT ethanolic extract and dilutions. The procedure prescribed in the monograph
of Indian Homeopathic pharmacopoeia was followed for the preparation of RT extract and its ultra-dilutions except the characteristic successions used in preparation of homeopathic dilutions. Dried and coarse powder of RT leaves was pulverized. Exactly weighed (10 gm) powder was mixed with 100mL of ethanol (70%) and kept in the glass jar for cold maceration up to 7 days with occasional shaking during each day36,54. On 8th day, mixture was fltered through Whatman flter paper and the fltrate was used as an alcoholic extract of RT. Various dilutions of extract in ethanol were prepared to obtain the fnal RT concentrations of 1×10−2, 1×10−4, 1×10−6, 1×10−8,
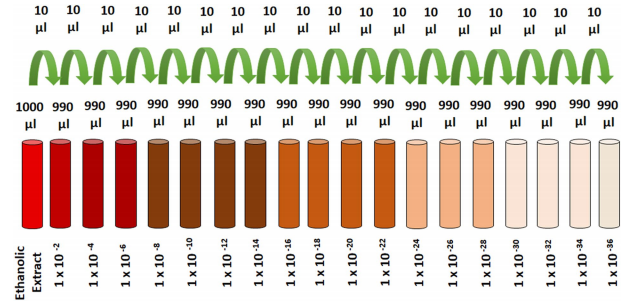
Figure 7. Schematics for the preparation of RT dilutions.
1×10−10, 1×10−12, 1×10−14, 1×10−16, 1×10−18, 1×10−20, 1×10−22, 1×10−24, 1×10−26, 1×10−28, 1×10−30, 1×10−32, 1×10−34, 1×10−36 (Fig. 7)
MTT cell viability assay. Te cytotoxicity study of RT in LPS mediated ROS-induced U-87 cells was performedusing MTT [3-(4,5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide] viability assay as described earlier5,35. Approximately, 1×104 cells were seeded in triplicate in 96-well tissue culture plates and allowed to reach 80% confuence. The U-87 cells were treated with 500ng/ml of LPS for 20minutes to induce ROS. Te LPS containing media was replaced with fresh media and the cells were treated with diferent concentrations of RT
(1×10−2–1×10−36) for further 24 h. Ten, MTT solutions (0.05 μg/μl) diluted in PBS was added to each well. The plates were incubated overnight at 37 °C to allow the formation of purple formazan crystals. Thereafer, detergent solution was added to each well to solubilise the crystals and incubated for 30min at 37°C. The intensity of formed color afer dissolving the formazan crystals in DMSO was measured spectrophotometrically using a microplate reader (Berthold Technologies, Germany) at 570nm. Each data point was performed in triplicate and all assays were executed thrice. Te data were represented as the percent (%) viability against control.
Cell culture and treatment. Te human glioblastoma cell line U-87 was maintained in Dulbecco’s modifed eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100U/ml penicillin, 100 μg/ml of streptomycin, 1.5mM of L-Glutamine in the humidifed atmosphere of 5% CO2 at 37 °C. Te U-87 cells were cultured in the culture fasks (75 cm2) and medium was changed at every alternate day. Afer 80% confuence, the media was replaced with fresh media containing LPS (500ng/mL) for 20min to induce the production of reactive oxygen species (ROS). Various concentrations of RT were added in LPS pre-treated cells for another 24h prior to perform the next experiments. The concentration of ethanol in the fnal assay medium wasless than 0.1%. The hydrogen peroxide (H2O2) at the fxed concentration (10 μM) was treated for 30min to produce ROS and used has positive control.
Determination of ROS, SOD, catalase activity and cytokines in U-87 cells. LPS pre-treated
(500ng/ml for 20min) U-87 cells were treated with diferent concentrations of RT before the estimation of ROS. Flow cytometry analysis of ROS production by 2′-7′ Dichlorodihydrofuorescein diacetate (DCFH-DA) staining were performed using fow cytometer (FACS Canto II, Becton & Dickinson, CA, USA) as described earlier by Eruslanov and Kusmartsev37 with some modifcations. Te H2O2 was used as positive control for the generation of ROS. Te concentration of SOD and catalase level in LPS-pretreated U-87 cells were measured using earlier reported methods38,39. Te quantifcation of cytokines including TNF- α, IL-1β, IL-6, IL-10 in LPS-pretreated U-87 cells were executed in cell culture supernatants using 50μg of protein by the commercially obtained ELISA kits as per the manufacturer’s instructions5,38.
Animals. Adult albino Wistar rats of either sex (170–220 g) were used for the present study. Animals were obtained from animal house facility of R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur. Animals were maintained in ventilated polypropylene cages under the standard conditions (25 ± 2 °C, 12 h light/ dark cycle) at the animal house facility of the institute. Animals were fed with standard pelletized feed (Nutrimix Std-1020) obtained from Nutrivet Life Sciences, Pune, India and water was provided ad libitum excluding the period of behavioral parameter evaluation. Te study was approved by the Institutional Animal Ethical Committee (Approval No. IAEC/RCPIPER/2016-17/02) of the R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur, India (Reg. No.651/PO/ReBi/S/02/CPCSEA). All the experimental procedures involving the use of animals were carried out in accordance with the regulations laid down by Committee for the Purpose of Control and Supervision of Experimentation on animals (CPCSEA) constituted under the Prevention of Cruelty to Animals Act, 1960, Ministry of Environment and Forests, Government of India.
Induction of chronic constriction injury (CCI) in rats. Te surgery was performed to induce the CCI as described earlier by Chanchal et al. 5. Briefy, the animals were anaesthetized with intraperitoneal administration of pentobarbital sodium (60mg/kg). A blunt dissection through biceps femoris was executed to expose the common sciatic nerve of right hind limb at the middle of the thigh. Approximately, 5–7 mm of the nerve was freed of the adhering tissue proximal to the trifurcation of sciatic nerve and four ligatures (6.0 silk) were loosely tied around it about 1 mm apart. Following nerve ligation, the muscular and skin layers were instantly sutured and povidone-iodine solution was applied externally. Te rats were kept in individual cages and allowed to recover29. The respective drug treatments were started on the next day after the surgery.
Drug treatment and groups. Animals were randomly divided into five groups, each consisting of 8 rats (n=8). Group I: Normal control group of rats were orally administered once daily with 1 ml saline for 14 days. Group II: Sham operated group of rats were treated with 1 ml saline once daily for 14 days. Group III: CCI-induced neuropathy control group of rats orally received 1 ml saline once daily for 14 days. Group IV: CCI-induced neuropathy+RT treated group of rats orally received 0.1 ml of RT (1×10−12 dilution) with 1 ml of distilled water once daily for 14 days. Group V: CCI-induced neuropathy+gabapentin treated group of rats orally received Gabapentin (60mg/kg/day, p.o.) suspended in 0.5% carboxymethyl cellulose (CMC) once daily for 14 days.
Experimental design. Subsequent to the induction of CCI, rats were habituated for 3days. RT treatment was started on the next day afer the CCI surgery. Te thermal and mechanical allodynia were measured on Day-3, Day-7, Day-11 and Day-14 following the surgery by earlier reported methods5,40. Paw withdrawal latency (PWL) was recorded with the maximum cut of time of 20 sec. Right hind paw of each rat up to the ankle joint was immersed in warm water (40±1°C) and cold water (12±1°C) for the determination of thermal (warm and cold) allodynia. Mechanical allodynia was noted using electronic Von-Frey apparatus comprising of super-tip probes (2390 series, IITC Life Sciences Incorporation). Paw withdrawal threshold (PWT) was recorded with cut-of pressure of 30 gm. Rats were kept in polypropylene cages with metal mesh foor and acclimatized for approximately 10min before the measurements. Mid-plantar surface of operated hind paw were probed with Von Frey flaments through the mesh foor, when the paw was in contact with foor. Each flament was applied to the planter surface until it just bent and kept in position for about 6–8 sec. Probes were applied in ascending order and the smallest flament which provoked paw withdrawal response was measured as threshold stimulus55. On the 14th day afer surgery, the animals were anesthetized with intraperitoneal injection of pentobarbital sodium (60 mg/kg). Te body temperature of animal was maintained at 37 °C. Sciatic-tibial motor nerve conduction velocity (MNCV) was measured by the stimulation of sciatic and tibial nerves at the sciatic notch and tibial notch through the bipolar needle electrodes (Power Lab/ML856; AD Instruments, Australia) at the 0.10Hz frequency, 0.1 ms duration and 1.5 V amplitude. Afer single stimulus the compound muscle action potential was measured from the frst interosseous muscle of the hind-paw by unipolar pin electrodes. The recording was typical biphasic response with an initial M-wave which is a direct motor response owing to stimulation of motor fbers. Te MNCV was calculated as the ratio of the distance (mm) between both sites of stimulation divided by the diference between proximal and distal latencies recorded in ms32,56. Following the recording of MNCV, the rats were sacrifced using overdose of pentobarbital sodium. The injured sciatic nerve was isolated along with 1 cm segments on the both sides of CCI injury. Te central 5 mm portion of the isolated nerve segment was processed for histological examination. Te sections of 4μm thickness were stained with haematoxylin and eosin. Te stained sections were examined under the light microscope for structural alterations including fber derangement, swelling of nerve fber and presence of activated satellite cells and Schwann cells. Paraformaldehyde-fxed nerve tissues were dehydrated in ascending graded series of alcohol and embedded in parafn. Te specimens were cut into the sections of 4 μm thickness using microtome and stained with hematoxylin and eosin according to routine staining protocols. Te stained sections were examined under the light microscope (Leica D1000, LED) for structural alterations like nerve fber swelling, fber derangement and presence of activated satellite cells and Schwann cells 13,57. Segments of sciatic nerve from the rats were process in ice chilled phosphate bufer (pH 7.4) to obtain the 10% homogenate. Homogenate was centrifuged at 2000 g for 20min at 4 °C and aliquots were used for the determination of malondialdehyde (MDA)58, reduced glutathione (GSH)59, superoxide dismutase (SOD)38 and catalase39, 60. Nitric oxide (NO) was estimated using earlier reported method by Kumar et al. 41 with some modifcations.
Briefy, 50 μl of tissue supernatant was mixed with 500 μl of Griess reagent and the absorbance was determined spectrophotometrically at 540nm using Powerwave XS microplate spectrophotometer (Biotek, USA). Calibration curve was obtained by using Sodium nitrite as a standard. Te concentration of NO was expressed in μM of NO per mg of protein. The quantifcation of cytokines like TNF- α, IL-1β and IL-6 was determined in homogenate and using 50μg of protein by the commercially obtained ELISA kits as per the manufacturer’s instructions.
